J INTS BIO, AACR 2024 - 'JIN-A02', a Novel Oral 4th Generation EGFR TKI, showing Clinical Benefits in the Ongoing Phase 1 Study
SEOUL, South Korea, April 11, 2024 /PRNewswire/ -- J INTS BIO announced the results of its ongoing Phase 1 clinical study of JIN-A02, a 4th generation EGFR-TKI for NSCLC treatment, at the 8th April poster session of the 2024 American Cancer Research Society (AACR) Annual Meeting held in San Diego, California, from 5th to 10th April (U.S. local time).
Professor Cho, Byoung Chul is having a conversation about the poster presentation of Phase 1/2 study of its Novel Oral 4th Generation EGFR-TKI ‘JIN-A02’ at the 2024 American Association of Cancer Research in San Diego, USA (AACR 2024)
'JIN-A02' is a 4th generation EGFR-TKI targeting C797S mutation that causes resistance to 3rd generation EGFR-TKI (such as Osimertinib, Lazertinib), commonly used in the treatment of NSCLC. 'JIN-A02' also targets T790M mutation independently of C797S mutation.
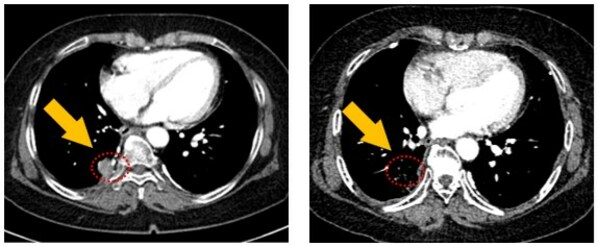
This ongoing Phase 1 clinical study is currently in its 4th Dose Level of 100mg daily and patients have showed increasing clinical benefits with each increasing dose levels. To-date, Partial Response (PR) was confirmed in one subject on the prior dose level of 50mg daily and stable diseases in two other subjects separately in Dose Level 50mg and 25mg daily.
Despite the initial low doses, JIN-A02 has showed clinical benefits immediately, cumulating in a Partial Response at the 3rd Dose Level of 50mg, which is an encouraging result in such an early stage of clinical development, the company explained. Furthermore, JIN-A02 has showed a favorable safety profile with no cardiotoxicity, rash or diarrhea reported, even at the current Dose Level of 100mg daily and the longest duration of treatment is over 8 months and still continuing, the company added. The 5th Dose Level of 150mg daily is expected to begin in 3Q 2024.
J INTS BIO confirmed the favorable safety and efficacy signals of 'JIN-A02' with clinical benefits in this poster presentation in San Diego and greater clinical impact is expected as the study progresses.
Featured Video
Biotechnology Recent Releases
- HanAll Biopharma Announces Initiation of Phase III Randomized, Double-Masked Vehicle Controlled VELOS-4 Trial Evaluating Tanfanercept for Treatment of Dry Eye Disease
- Bio Basic Asia Pacific Offers Free Re-Sequencing and Gel Analysis for Sanger Sequencing Services
- Clarivate Enhances Cortellis CMC Intelligence with Post-Approval Module to Accelerate Regulatory Success
- Integrated cancer biotech Infinitopes secures £12.8m seed financing to enhance its Precision Immunomics™ antigen discovery technologies to target five more cancers
- Neogen® Petrifilm® Celebrates 40 Years!
- Read more
Health Care/Hospital Recent Releases
- VYNDAMAX® (tafamidis) PBS-listed for adult patients with wild-type or hereditary transthyretin amyloid cardiomyopathy (ATTR-CM) with New York Heart Association (NYHA) Class I-II heart failure
- United Imaging to Unveil Industry-Leading MRI Advancements at ISMRM 2024
- HanAll Biopharma Announces Initiation of Phase III Randomized, Double-Masked Vehicle Controlled VELOS-4 Trial Evaluating Tanfanercept for Treatment of Dry Eye Disease
- Hang Seng Insurance Introduces FortuneLife Deferred Annuity Life Insurance Plan with Enhanced Benefit to Support Stable Retirement
- Zepp Health's Amazfit Unveils Bip 5 Unity: Elevating Health and Style Every Step of the Way
- Read more
Medical/Pharmaceuticals Recent Releases
- VYNDAMAX® (tafamidis) PBS-listed for adult patients with wild-type or hereditary transthyretin amyloid cardiomyopathy (ATTR-CM) with New York Heart Association (NYHA) Class I-II heart failure
- United Imaging to Unveil Industry-Leading MRI Advancements at ISMRM 2024
- HanAll Biopharma Announces Initiation of Phase III Randomized, Double-Masked Vehicle Controlled VELOS-4 Trial Evaluating Tanfanercept for Treatment of Dry Eye Disease
- Clarivate Enhances Cortellis CMC Intelligence with Post-Approval Module to Accelerate Regulatory Success
- Vivo Surgical Announces Leading Gastrointestinal Endoscopists on Clinical Advisory Board for Novel Endoscopic Robot
- Read more
Pharmaceuticals Recent Releases
- VYNDAMAX® (tafamidis) PBS-listed for adult patients with wild-type or hereditary transthyretin amyloid cardiomyopathy (ATTR-CM) with New York Heart Association (NYHA) Class I-II heart failure
- HanAll Biopharma Announces Initiation of Phase III Randomized, Double-Masked Vehicle Controlled VELOS-4 Trial Evaluating Tanfanercept for Treatment of Dry Eye Disease
- Everest Medicines Announces Hong Kong Department of Health Approval of Nefecon® for the Treatment for Primary IgA Nephropathy in Adult Patients
- 3Shape Unveils Unite 3rd Generation: Access TRIOS Scans Anywhere, Anytime
- ArisGlobal Helps Boehringer Ingelheim Transform Safety Signal Processing by Leveraging Latest LifeSphere Solutions
- Read more